From Section 5, part c) Synthetic polymers
5.14 recall that an addition polymer is formed by joining up many small molecules called monomers
5.14 recall that an addition polymer is formed by joining up many small molecules called monomers
5.15 draw the repeat unit of addition polymers, including poly(ethene), poly(propene) and poly(chloroethene)
5.16 deduce the structure of a monomer from the repeat unit of an addition polymer
Monomers are small molecules that can join up to make a very large molecule called a polymer.
e.g. Starch is a polymer made up of small glucose monomers
The monomer in addition polymerisation is unsaturated, i.e. the monomer must have a double bond. This opens up to allow other monomers to join up to form the polymer.
Watch- Polymers and Monomers made easy, it's a bit long, about 7 minutes, but it's definitely clear and precise.
If the video doesn't come up here, go to youtube, type
If the video doesn't come up here, go to youtube, type
Polymers and Monomers BBLC, and watch!
 |
| Repeat unit of ethene, excuse my poor 'paint' skills, I wish I had a tablet! |
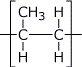 |
| Repeat unit of propene |
According to my teacher, you shouldn't write the 'n' for the repeat unit, only for the polymer to show that there are many more monomers attached to the section you have drawn like the following picture, a lot of the repeat unit for chloroethene makes poly(chloroethene)-obviously you're not going to draw a thousand chloroethene monomers for polychloroethene..
 |
| 'n' represents a large but variable number. It simply means that the structure in the bracket repeats itself many times in the molecule. |
To deduce the structure of a monomer from the repeating unit or the structure of the monomer, first find the repeat unit, and then put back the original carbon-carbon double bond. (because this opened up to form the repeat unit, whereby other monomers would join end to end as their bonds open up as well). To find the name, count how many carbons there are in the repeat unit.
The reason why the repeat units only show 2 carbons is because we're showing what happened to the double bonds. So even if the monomer is pentene with 5 carbons, your repeating unit shows 2 carbons with 3 hydrogen atoms attached and a C3H7 on the remaining branch, as pentene is C5H10. And please don't forget that there is no double bond in the repeating unit, it has opened up so don't forget these bonds on the ends with a bracket around it too. :)
The reason why the repeat units only show 2 carbons is because we're showing what happened to the double bonds. So even if the monomer is pentene with 5 carbons, your repeating unit shows 2 carbons with 3 hydrogen atoms attached and a C3H7 on the remaining branch, as pentene is C5H10. And please don't forget that there is no double bond in the repeating unit, it has opened up so don't forget these bonds on the ends with a bracket around it too. :)
An update for people still confused about addition polymerisation:
Ethene is one of the alkenes produced by cracking. It is the smallest hydrocarbon containing a carbon-carbon double bond. (Carbon-carbon double bonds obviously need 2 carbons, and ethene has 2. In alkanes it may start with methane, but there is no such thing as methene, as with one carbon atom only, a carbon-carbon double bond cannot exist.)
Under the right conditions, molecules contain carbon-carbon double bonds can join together to produce very long chains. Part of the double bond is broken, it 'opens up', and the electrons in it are used to join to neighbouring molecules. This is called addition polymerisation. (Don't worry too much about the electrons bit, it's just to help you understand how.)
Polymerisation is the joining up of lots of little molecules (the monomers) to make one big molecule (the polymer). In the case of ethene, lots of ethene molecules join together to make poly(ethene).
 |
| Three ethene monomers |
 |
| Double bonds 'opening up' |
 |
| Joining of monomers to form long carbon chain-poly(ethene) |
The chain length can vary from about 4000 to 40,000 carbon atoms. For normal purposes, this is written using displayed formulae.

propene (monomer) → poly(propene) (polymer)
The monomers used to make addition polymers are based on ethene, for e.g. propene.
 |
Although this is the usual way to draw the structural formula for propene, for the purposes of showing how the molecule acts as a monomer and can form a polymer it should be drawn in a different way: |
 |
| Chloroethene monomer. Now you try to work out how it polymerises. Draw three of these monomers, open up the double bonds, and join them together! |
If the structure of the polymer is given then the structure of the monomer can be worked out.
 |
| Look for the repeating unit i.e. the part of the molecule that is repeated. |
 |
| The repeating unit should be made up of two carbon atoms from the main. |
 |
| Now put a double bond between the two carbon atoms to get the monomer. |
Typed from an edexcel book:
Uses for poly(ethene):
Poly(ethene) comes in two types: low-density poly(ethene) (LDPE) and high-density poly(ethene) (HDPE). Low-density poly(ethene) is mainly used as a thin film to make polythene bags. It is very flexible and not very strong. E.g. those supermarket plastic bags
High-density poly(ethene) is used where greater strength and rigidity is needed-for example to make plastic bottles such as milk bottles. If you can find a recycling symbol with the letters HDPE next to it, then the bottle is made of high-density poly(ethene). If it has some other letters there, then it is a different polymer.
Uses of poly(propene):
Poly(propene) is somewhat stronger than poly(ethene). It is used to make ropes and crates (among many other things). If an item has a recycling mark with PP inside it or near it, it is made of poly(propene).
Uses of poly(chloroethene):
Poly(chloroethene)-PVC-has a lot of uses. It is quite strong and rigid, and so can be used for drainpipes or replacement windows. It can also be made flexible by adding 'plasticisers'. That makes it useful for sheet floor coverings, and even clothing. These polymers don't conduct electricity, and PVC is used for electrical insulation. It is now replacing rubber to insulate wires as it doesn't crack so easily, thus it's safer. (My physics blog talks about electricity and its dangers.)
.gif)



section 1 e) Chemical formulae... please :)
ReplyDeletehi. I hope you can explain the specification 5.17-5.20. Its not in the double award specification but single award. I hope it is not a problem.thank you.
ReplyDeleteHey so I've tried to explain it in a new post 'Condensation polymerisation-Nylon'.
DeleteI hope it helps! Single science is much harder to explain and understand.. :S so tell me how it goes. :)
your explaination was good. I liked the video about additional vs codensation polymerisation, it was useful.
DeleteThis site is so helpful thank you so much! :)
ReplyDeleteThankss and you're welcome. :D
Deletei LOVE THIS SITE <3
ReplyDeletehey could u pls help with specification 3.2-3.8 organic chemistry :) hehe thx
ReplyDeleteThank you for all this info...love it! it helped clear up so many of my doubts, and i love the fact that it is so summerised ^.^
ReplyDeleteReally nice breakdown, I have heard the good that things like PEGylated polymers have been doing in the realm of science lately and it nice to finally learn a bit of what is going on
ReplyDelete